SkipGNN: Predicting Molecular Interactions with Skip-Graph Networks
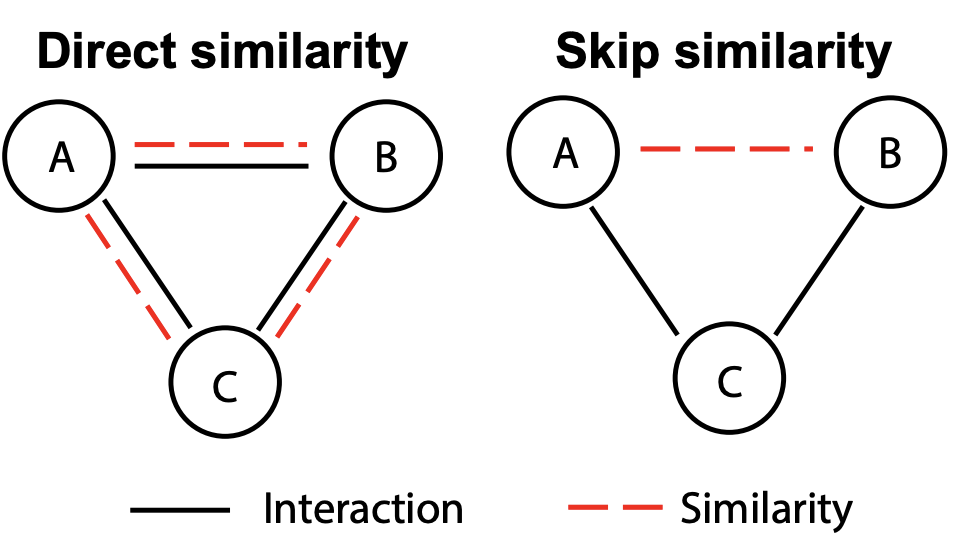
Molecular interaction networks are powerful resources for the discovery. They are increasingly used with machine learning methods to predict biologically meaningful interactions. While deep learning on graphs has dramatically advanced the prediction prowess, current graph neural network (GNN) methods are optimized for prediction on the basis of direct similarity between interacting nodes. In biological networks, however, similarity between nodes that do not directly interact has proved incredibly useful in the last decade across a variety of interaction networks. Here, we present SkipGNN, a graph neural network approach for the prediction of molecular interactions. SkipGNN predicts molecular interactions by not only aggregating information from direct interactions but also from second-order interactions, which we call skip similarity. In contrast to existing GNNs, SkipGNN receives neural messages from two-hop neighbors as well as immediate neighbors in the interaction network and non-linearly transforms the messages to obtain useful information for prediction. To inject skip similarity into a GNN, we construct a modified version of the original network, called the skip graph. We then develop an iterative fusion scheme that optimizes a GNN using both the skip graph and the original graph. Experiments on four interaction networks, including drug-drug, drug-target, protein-protein, and gene-disease interactions, show that SkipGNN achieves superior and robust performance, outperforming existing methods by up to 28.8\% of area under the precision recall curve (PR-AUC). Furthermore, we show that unlike popular GNNs, SkipGNN learns biologically meaningful embeddings and performs especially well on noisy, incomplete interaction networks.
PDF Abstract